
In both types of sheet, the beta-strands are parallel to each other.Ĭlick on the highlighted regions of the plot to show the residues in the structure. The strands can run in the same direction, generating a parallel beta-sheet, or they can run in opposite directions, generating an anti-parallel beta sheet. In a beta-sheet there are two possibilities for the relative orientation of the individual strands. To color the beta-carbon of each residue cyan. In a beta-sheet, the sidechains are found in the plane of the sheet.

(both strands run in the same direction.)ģ. To highlight the amino-terminus and the carboxy-terminus of each strand.Ī. Is the beta-sheet parallel or anti-parallel? (a. Although both atoms are of the correct type, the electropositive proton is not close to the electronegative acceptor b. Although both atoms are of the correct type, the electropositive proton is not close to the electronegative acceptor Although both atoms are of the correct type, the geometry is incorrect - hydrogen bonds are linear.)Ģ. Which of the labeled atoms can be hydrogen bond donors and which can be acceptors? See donors (grey). See acceptors (orange). In the Jmol image of the beta sheet, which of the following pairs of atoms participate in a hydrogen bond? Use this Jmol structure to answer the questions.ġ. The amino acid sidechains are also directed outward from the strand and alternate between pointing upwards and downwards with respect to the plane of the sheet. Consequently H-bonds are between adjacent strands and perpendicular to the direction of the strand. Two or more beta-strands make a beta-sheet. Due to the extended nature of the polypeptide it is not possible to form mainchain hydrogen bonds between adjacent residues. Consequently, beta structures consist of straight, fully extended, strands of linked amino acids which are called beta-strands. These particular phi and psi torsional angles generate a building block that is almost a perfect rectangular prism. In all cases each peptide bond is rigid and planar in the trans conformation. In all cases these secondary structures of proteins have characteristic values of the \(\Psi\) and \(\Phi\) torsional angles that are the same for each residue within a particular secondary structure.
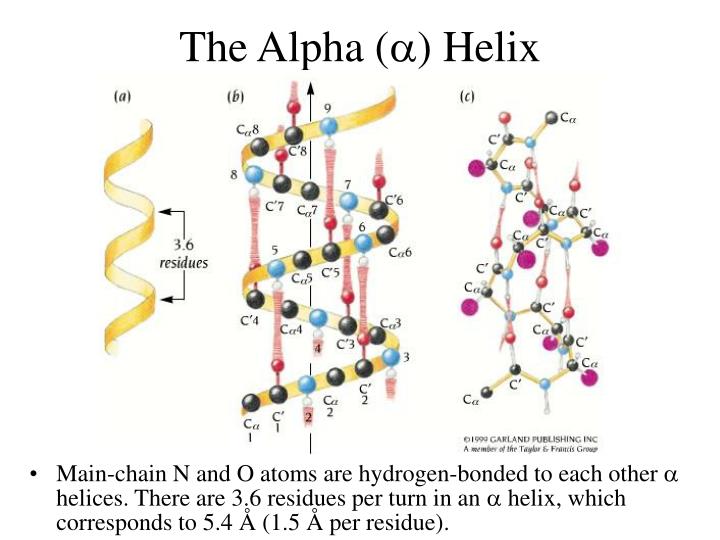
#Alpha helix protein backbone series#
If a series of identically shaped objects are laid end-to-end they will form some type of geometrical structure. In regular secondary structures these angles are the same for each residue, and thus the shape of each building block, or amino acid, is the same. The shape of each block depends on the \(\Phi\) and \(\Psi\) angle of each residue. Proteins consist of a linear chain of amino acids, with each amino acid representing a build block.


 0 kommentar(er)
0 kommentar(er)
